Currently, during birth there are many potential disruptions to the healthy development of the infant's microbial ecosystem. Some practices to be concerned about: the use of antibiotics during pregnancy and during delivery, c-sections, newborns routinely given antibiotics, and then bottle feeding instead of breastfeeding. Sometimes one or more of these practices are medically necessary, but currently they are being done much too frequently and casually. In these ways we are conducting an experiment on every baby's microbial ecosystem with unknown long-term consequences. The following excerpts from Dr.Martin Blaser's popular 2014 book Missing Microbes: How the Overuse of Antibiotics Is Fueling Our Modern Plagues, even though written a year ago, are a nice summary of these issues. From Wired:
The Way You’re Born Can Mess With the Microbes You Need to Survive
THROUGHOUT THE ANIMAL kingdom, mothers transfer microbes to their young while giving birth....And for millennia, mammalian babies have acquired founding populations of microbes by passing through their mothers’ vagina. This microbial handoff is also a critical aspect of infant health in humans. Today it is in peril.
Microbes play a hidden role in the course of every pregnancy. During the first trimester, certain species of bacteria become overrepresented while others become less common. By the third trimester, just before the baby is born, even greater shifts occur. These changes, involving scores of species, are not random. The compositions change in the same direction across the dozens of women who have been studied.... Women of reproductive age carry bacteria, primarily lactobacilli, which make the vaginal canal more acidic. This environment provides a hardy defense against dangerous bacteria that are sensitive to acid. Lactobacilli also have evolved a potent arsenal of molecules that inhibit or kill other bacteria.
Whether the birth is fast or slow, the formerly germ-free baby soon comes into contact with the lactobacilli. The baby’s skin is a sponge, taking up the vaginal microbes rubbing against it. The first fluids the baby sucks in contain mom’s microbes, including some fecal matter.
Once born, the baby instinctively reaches his mouth, now full of lactobacilli, toward his mother’s nipple and begins to suck. The birth process introduces lactobacilli to the first milk that goes into the baby. This interaction could not be more perfect. Lactobacilli and other lactic acid–producing bacteria break down lactose, the major sugar in milk, to make energy. The baby’s first food is a form of milk called colostrum, which contains protective antibodies. The choreography of actions involving vagina, baby, mouth, nipple, and milk ensures that the founding bacteria in the baby’s intestinal tract include species that can digest milk for the baby.
Breast milk, when it comes in a few days later, contains carbohydrates, called oligosaccharides, that babies cannot digest. But specific bacteria such as Bifidobacterium infantis, another foundational species in healthy babies, can eat the oligosaccharides. The breast milk is constituted to give favored bacteria a head start against competing bacteria.
Cesarian delivery is a largely unrecognized threat to the microbial handoff from mother to child. Instead of traveling down the birth canal picking up lactobacilli, the baby is surgically extracted from the womb through an incision in the abdominal wall....For all of these reasons, U.S. C-section rates increased from fewer than one in five births in 1996 to one in three births in 2011—a 50 percent increase.
The founding populations of microbes found on C-section infants are not those selected by hundreds of thousands of years of human evolution. A few years ago in Puerto Ayacucho, Venezuela, my wife, Gloria, conducted the first study of its kind to test whether the microbes found on newborn babies delivered vaginally or by C-section varied in any way....The mouths, skin, and first bowel movements of babies born vaginally were populated by their mother’s vaginal microbes: Lactobacillus, Prevotella, or Sneathia species. Those born by C-section harbored bacterial communities found on skin, dominated by Staphylococcus, Corynebacterium, and Propionibacterium.
In other words, their founding microbes bore no relationship to their mother’s vagina or any vagina. At all the sites—mouth, skin, gut—their microbes resembled the pattern on human skin and organisms floating in the air in the surgery room. They were not colonized by their mother’s lactobacilli. The fancy names of these bacteria don’t matter as much as the notion that the founding populations of microbes found on C-section infants are not those selected by hundreds of thousands of years of human evolution or even longer.
Another threat to a baby’s newly acquired resident microbes involves antibiotics given to the mother. Most doctors consider it safe to prescribe penicillins for all sorts of mild infections in pregnancy—coughs, sore throats, urinary tract infections. Sometimes when doctors think that the mother has a viral infection they also give antibiotics just in case it is actually a bacterial infection.
Then comes the birth itself. Women in labor routinely get antibiotics to ward off infection after a C-section....Antibiotics are broad in their effects, not targeted....The problem, of course, is that we know antibiotics are broad in their effects, not targeted. While the antibiotic kills Group B strep, it also kills other often-friendly bacteria, thus selecting for resistant ones. This practice is altering the composition of the mother’s microbes in all compartments of her body just before the intergenerational transfer is slated to begin.
The baby also is affected in similar unintended ways. Any antibiotic that gets into the bloodstream of the fetus or into the mother’s milk will inevitably influence the composition of the baby’s resident microbes, but we are only beginning to understand what this means.
Finally, the babies are directly exposed. Most parents are not aware that all American-born babies today are given an antibiotic immediately after birth. The reason is that many years ago, before antibiotics, women who unknowingly had gonorrhea would pass the infection to their babies, giving the newborns terrible eye infections that could cause blindness...The dose is low but is likely affecting the composition of the infant’s resident microbes just when the founding populations are developing. We should be able to develop a better way to screen, so we can target those babies at the highest risk, perhaps a few hundred among the millions of births a year.
Although babies are born into a world replete with diverse bacteria, the ones that colonize them are not accidental. These first microbes colonizing the newborn begin a dynamic process. We are born with innate immunity, a collection of proteins, cells, detergents, and junctions that guard our surfaces based on recognition of structures that are widely shared among classes of microbes. In contrast, we must develop adaptive immunity that will clearly distinguish self from non-self. Our early-life microbes are the first teachers in this process, instructing the developing immune system about what is dangerous and what is not.
 A newborn infant, seconds after delivery. Amniotic fluid glistens on the child's skin. Credit: Wikipedia, Ernest F
A newborn infant, seconds after delivery. Amniotic fluid glistens on the child's skin. Credit: Wikipedia, Ernest F
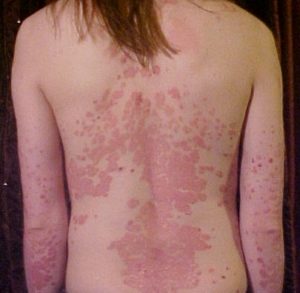 Could probiotics have a role to play in the treatment of psoriasis? A recent analysis and review of studies suggests that they might. Psoriasis is a non-contagious, chronic disease affecting about 2 to 4% of the population, and which is characterized by patches of abnormal skin. These skin patches are typically red, itchy, and scaly, and can cover small areas to covering the entire body. There is no cure for psoriasis, but various treatments can help control the symptoms, such as steroid creams, vitamin D3 cream, ultraviolet light, and immune system suppressing medications.
Could probiotics have a role to play in the treatment of psoriasis? A recent analysis and review of studies suggests that they might. Psoriasis is a non-contagious, chronic disease affecting about 2 to 4% of the population, and which is characterized by patches of abnormal skin. These skin patches are typically red, itchy, and scaly, and can cover small areas to covering the entire body. There is no cure for psoriasis, but various treatments can help control the symptoms, such as steroid creams, vitamin D3 cream, ultraviolet light, and immune system suppressing medications. 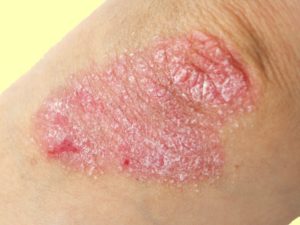 Psoriasis. Credit: Medscape
Psoriasis. Credit: Medscape
