The CDC currently recommends only consuming pasteurized or ultra-pasteurized milk and dairy products. This is because heating milk kills off the bird flu virus (H5N1) now circulating among dairy cows in the USA. Another serious virus that pasteurization and ultra-pasteurization of milk kills off is the tick-borne encephalitis that occurs throughout central and eastern Europe.
The CDC currently recommends only consuming pasteurized or ultra-pasteurized milk and dairy products. This is because heating milk kills off the bird flu virus (H5N1) now circulating among dairy cows in the USA. Another serious virus that pasteurization and ultra-pasteurization of milk kills off is the tick-borne encephalitis that occurs throughout central and eastern Europe.
Tick bites are the major way tick-borne encephalitis is spread. But a minority of cases are spread by consuming raw milk or dairy products from recently infected livestock (goats, sheep, and cows).
 Tick-borne encephalitis is a serious viral infection of the central nervous system. It starts out with symptoms such as fever, headaches, chills, but up to 39% of cases result in more serious neurological symptoms (meningitis, encephalitis). Infected persons may experience long-term neurological effects lasting years.
Tick-borne encephalitis is a serious viral infection of the central nervous system. It starts out with symptoms such as fever, headaches, chills, but up to 39% of cases result in more serious neurological symptoms (meningitis, encephalitis). Infected persons may experience long-term neurological effects lasting years.
Two ways to avoid the tick-borne encephalitis virus: only consuming pasteurized milk and dairy products (and avoiding raw milk and dairy products) or getting vaccinated with the tick-borne encephalitis vaccine (TicoVac, TBE vaccine).
Bottom line: Only consume pasteurized or ultra-pasteurized milk and dairy products.
From article (page 3) in Medscape: Fast Five Quiz: Diagnose and Treat Tick-Borne Illnesses
Tick-borne encephalitis virus can be transmitted by ticks and the alimentary tract. A recent meta-analysis of 410 foodborne tick-borne encephalitis cases, mostly from a region in central and eastern Europe, aimed to describe cases of tick-borne encephalitis acquired through alimentary transmission in Europe from 1980 to 2021. ...continue reading "Raw Milk In Europe May Contain A Serious Virus"

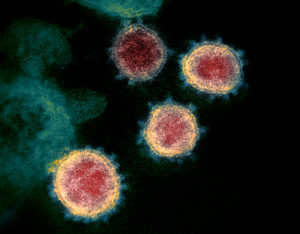
 The bird flu virus (H5N1 virus) has now been found in a number of American dairy herds and in raw milk from infected cows. Thus the medical advice is to
The bird flu virus (H5N1 virus) has now been found in a number of American dairy herds and in raw milk from infected cows. Thus the medical advice is to 




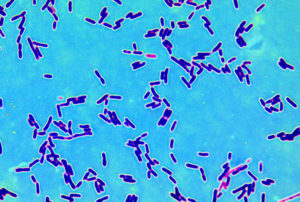
 Ever wonder what bacteria are living on your kitchen surfaces, including sponges? It turns out that even with different hygiene, dietary habits, and cooking practices, there is a core group of bacteria that are common to all kitchen surfaces (core microbiota). At least this was true for residential kitchens in 5 European countries.
Ever wonder what bacteria are living on your kitchen surfaces, including sponges? It turns out that even with different hygiene, dietary habits, and cooking practices, there is a core group of bacteria that are common to all kitchen surfaces (core microbiota). At least this was true for residential kitchens in 5 European countries.