 The bottom line is to read the ingredients list on products, and avoid all products labeled "antimicrobial" or "antibacterial" (because those are the ones typically containing triclosan and triclorocarban). Over 2000 products contain antibacterial compounds. I've even seen them in pillows, pillow protectors, mattress pads, dish racks, toys, and blankets! As we know from the latest microbiology research, we need to cultivate a healthy microbiome, and not throw it out of whack by continuously trying to kill off all bacteria. From The Atlantic:
The bottom line is to read the ingredients list on products, and avoid all products labeled "antimicrobial" or "antibacterial" (because those are the ones typically containing triclosan and triclorocarban). Over 2000 products contain antibacterial compounds. I've even seen them in pillows, pillow protectors, mattress pads, dish racks, toys, and blankets! As we know from the latest microbiology research, we need to cultivate a healthy microbiome, and not throw it out of whack by continuously trying to kill off all bacteria. From The Atlantic:
It's Probably Best to Avoid Antibacterial Soaps
Antimicrobial chemicals are so ubiquitous that a recent study found them in pregnant mothers' urine and newborns' cord blood. Research shows that their risks may outweigh their benefits.
Antimicrobial chemicals, intended to kill bacteria and other microorganisms, are commonly found in not just soaps, but all kinds of products—toothpaste, cosmetics, and plastics among them. There is evidence that the chemicals aren’t always effective, and may even be harmful, and their ubiquity means people are often continually exposed to them. One such chemical, triclosan, has previously been found in many human bodily fluids. New research found traces of triclosan, triclocarban, and butyl paraben in the urine of pregnant women, and the cord blood of newborn infants.
The research looked at the same population of 180 expectant mothers living in Brooklyn, New York, most of Puerto Rican descent. In a study published last week in Environmental Science and Technology, researchers from Arizona State University and State University of New York’s Downstate School of Public Health found triclosan in 100 percent of the women’s urine samples, and triclocarban in 87 percent of the samples. Of the 33 cord blood samples they looked at, 46 percent contained triclosan and 23 percent contained triclocarban.
In another, still-unpublished study, the researchers found that all of the cord blood samples contained “at least one paraben,” according to Dr. Rolf Halden, director of ASU’s Center for Environmental Security.
Triclosan and triclocarban are endocrine disruptors, Halden explains. The risk there is that the chemicals can mimic thyroid hormones, potentially disrupting the metabolism and causing weight gain or weight loss. Previous research has also shown a connection between higher levels of triclosan in urine, and allergy diagnoses in children.
In the study looking at butyl paraben, the researchers found an association between higher exposure to the chemical, and a smaller head circumference and length of babies after they were born. Butyl paraben is used as a preservative, so it’s found in a wider breadth of products, according to Halden.
From Science News: Pregnant women, fetuses exposed to antibacterial compounds face potential health risks
As the Food and Drug Administration mulls over whether to rein in the use of common antibacterial compounds that are causing growing concern among environmental health experts, scientists are reporting that many pregnant women and their fetuses are being exposed to these substances. The compounds are used in more than 2,000 everyday products marketed as antimicrobial, including toothpastes, soaps, detergents, carpets, paints, school supplies and toys, the researchers say.
The problem with this, explains Pycke, a research scientist at Arizona State University (ASU), is that there is a growing body of evidence showing that the compounds can lead to developmental and reproductive problems in animals and potentially in humans. Also, some research suggests that the additives could contribute to antibiotic resistance, a growing public health problem.
Although the human body is efficient at flushing out triclosan and triclocarban, a person's exposure to them can potentially be constant. "If you cut off the source of exposure, eventually triclosan and triclocarban would quickly be diluted out, but the truth is that we have universal use of these chemicals, and therefore also universal exposure," says Rolf Halden, Ph.D., the lead investigator of the study at ASU.
 Everyone is concerned with the problem of antibiotics not working due to antibiotic resistance, that is, when bacteria resist the effects of antibiotics. Researchers typically study genetic changes that occur in bacteria over time, but researchers at Newcastle University in the UK found evidence for a another reason that antibiotics may not work in treating an infection. They found that bacteria can change shape and shed their cell walls, which are their outermost defense and the primary target of most antibiotics. Then when the antibiotics are stopped, they can go back to their original shape. Sneaky!
Everyone is concerned with the problem of antibiotics not working due to antibiotic resistance, that is, when bacteria resist the effects of antibiotics. Researchers typically study genetic changes that occur in bacteria over time, but researchers at Newcastle University in the UK found evidence for a another reason that antibiotics may not work in treating an infection. They found that bacteria can change shape and shed their cell walls, which are their outermost defense and the primary target of most antibiotics. Then when the antibiotics are stopped, they can go back to their original shape. Sneaky!
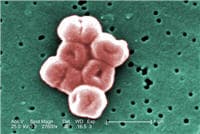 Many posts on this blog are about beneficial microbes, and the many species of microbes (bacteria, fungi, viruses) living within and on us. But there are also bacteria in the world that pose a serious threat to human health, and the list of these are growing due to antibiotic resistance. This week the World Health Organization (WHO) officials came out with a list of a dozen antibiotic-resistant "priority pathogens" that pose the greatest threats to human health. These are bacteria resistant to multiple antibiotics - thus superbugs.
Many posts on this blog are about beneficial microbes, and the many species of microbes (bacteria, fungi, viruses) living within and on us. But there are also bacteria in the world that pose a serious threat to human health, and the list of these are growing due to antibiotic resistance. This week the World Health Organization (WHO) officials came out with a list of a dozen antibiotic-resistant "priority pathogens" that pose the greatest threats to human health. These are bacteria resistant to multiple antibiotics - thus superbugs.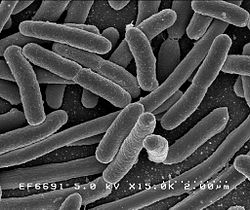 A few days ago the CDC (Centers for Disease Control and Prevention) released
A few days ago the CDC (Centers for Disease Control and Prevention) released  The big scary question: What will happen after antibiotics cease to work? And people start dying by the millions from infections that used to be easily treated? We are fast approaching that point of total antibiotic resistance, with superbugs that resist all antibiotics. More and more disease-causing bacteria are rapidly evolving immunity to every existing antibiotic (see
The big scary question: What will happen after antibiotics cease to work? And people start dying by the millions from infections that used to be easily treated? We are fast approaching that point of total antibiotic resistance, with superbugs that resist all antibiotics. More and more disease-causing bacteria are rapidly evolving immunity to every existing antibiotic (see  The following article is interesting because it describes how microbes are high up in the sky riding air currents and winds to circle the earth, and eventually drop down somewhere. This is one way
The following article is interesting because it describes how microbes are high up in the sky riding air currents and winds to circle the earth, and eventually drop down somewhere. This is one way  New research found that one course of
New research found that one course of  The bottom line is to read the ingredients list on products, and avoid all products labeled "antimicrobial" or "antibacterial" (because those are the ones typically containing triclosan and triclorocarban). Over 2000 products contain antibacterial compounds. I've even seen them in pillows, pillow protectors, mattress pads, dish racks, toys, and blankets! As we know from the latest microbiology research, we need to cultivate a healthy microbiome, and not throw it out of whack by continuously trying to kill off all bacteria. From The Atlantic:
The bottom line is to read the ingredients list on products, and avoid all products labeled "antimicrobial" or "antibacterial" (because those are the ones typically containing triclosan and triclorocarban). Over 2000 products contain antibacterial compounds. I've even seen them in pillows, pillow protectors, mattress pads, dish racks, toys, and blankets! As we know from the latest microbiology research, we need to cultivate a healthy microbiome, and not throw it out of whack by continuously trying to kill off all bacteria. From The Atlantic: Even though this study was done in a laboratory, it gives further support for the treatment of sinusitis with bacteria and other microbes. And it could help explain why repeated courses of antibiotics don't "cure" many chronic infections - because biofilms filled with pathogenic bacteria are signs of microbial communities out-of-whack. Which is why my family's successful chronic sinusitis treatment with kimchi (juice) containing Lactobacillus sakei is all the more impressive. From Science Daily:
Even though this study was done in a laboratory, it gives further support for the treatment of sinusitis with bacteria and other microbes. And it could help explain why repeated courses of antibiotics don't "cure" many chronic infections - because biofilms filled with pathogenic bacteria are signs of microbial communities out-of-whack. Which is why my family's successful chronic sinusitis treatment with kimchi (juice) containing Lactobacillus sakei is all the more impressive. From Science Daily: