 Two studies (one in mice and one in humans) by researchers at the University of Illinois found that no matter what your diet - exercise changes the gut bacteria in a beneficial way. And when you go back to a sedentary lifestyle, your gut microbes change again and beneficial microbes such as short chain fatty acids (SCFAs), especially butyrates, decline. The effect was more pronounced in lean sedentary adults (as compared to obese sedentary adults).
Two studies (one in mice and one in humans) by researchers at the University of Illinois found that no matter what your diet - exercise changes the gut bacteria in a beneficial way. And when you go back to a sedentary lifestyle, your gut microbes change again and beneficial microbes such as short chain fatty acids (SCFAs), especially butyrates, decline. The effect was more pronounced in lean sedentary adults (as compared to obese sedentary adults).
Beneficial microbes that increased with exercise in humans were species of Faecalibacterium, Roseburia, Lachnospira, Lachnospiraceae, and Clostridiales. Faecalibacterium prausnitzii has been discussed in earlier posts as a beneficial keystone species in the gut (here, here, and here).
What kind of exercises did they do? They did three supervised 30 to 60 minute moderate to vigorous intensity aerobic/endurance exercise sessions per week for 6 weeks, and they could use a cycle ergometer (stationary bicycle) or treadmill each session.
Besides beneficial microbial changes, 6 weeks of exercising resulted in improved body composition (total lean body mass, decreased body fat, increased bone mineral density), and an improvement in cardiorespiratory fitness. These changes reversed in everyone when they went back to 6 weeks of a sedentary lifestyle.
Bottom line: get out and move, move, move. Your gut microbes and your body will thank you.
From Science Daily: Exercise changes gut microbial composition independent of diet, team reports
Two studies -- one in mice and the other in human subjects -- offer the first definitive evidence that exercise alone can change the composition of microbes in the gut. The studies were designed to isolate exercise-induced changes from other factors -- such as diet or antibiotic use -- that might alter the intestinal microbiota.
In the first study, scientists transplanted fecal material from exercised and sedentary mice into the colons of sedentary germ-free mice, which had been raised in a sterile facility and had no microbiota of their own. In the second study, the team tracked changes in the composition of gut microbiota in human participants as they transitioned from a sedentary lifestyle to a more active one -- and back again.
Recipients of the exercised mouse microbiota also had a higher proportion of microbes that produce butyrate, a short-chain fatty acid that promotes healthy intestinal cells, reduces inflammation and generates energy for the host. They also appeared to be more resistant to experimental ulcerative colitis, an inflammatory bowel disease.
In the human study, the team recruited 18 lean and 14 obese sedentary adults, sampled their gut microbiomes, and started them on an exercise program during which they performed supervised cardiovascular exercise for 30-60 minutes three times a week for six weeks. The researchers sampled participants' gut microbiomes again at the end of the exercise program and after another six weeks of sedentary behavior. Participants maintained their usual diets throughout the course of the study. Fecal concentrations of SCFAs, in particular butyrate, went up in the human gut as a result of exercise. These levels declined again after the participants reverted to a sedentary lifestyle.
The most dramatic increases were seen in lean participants, who had significantly lower levels of SCFA-producing microbes in their guts to begin with. Obese participants saw only modest increases in the proportion of SCFA-producing microbes. The ratios of different microbes in the gut also differed between lean and obese participants at every stage of the study, the researchers said. "The bottom line is that there are clear differences in how the microbiome of somebody who is obese versus somebody who is lean responds to exercise," Woods said. " [Original study in humans.]

 Another
Another  A while ago I posted the results of studies showing differences in infant microbiomes (community of microbes) depending on whether the babies were delivered
A while ago I posted the results of studies showing differences in infant microbiomes (community of microbes) depending on whether the babies were delivered  The following is a nice article about a recently
The following is a nice article about a recently  A number of recent studies have suggested that as people age, the community of gut microbes (gut microbiota or gut microbiome) becomes less diverse than in younger people. And note that greater gut microbial diversity is generally viewed as healthy and good. However, now a study done in China finds a different result. The
A number of recent studies have suggested that as people age, the community of gut microbes (gut microbiota or gut microbiome) becomes less diverse than in younger people. And note that greater gut microbial diversity is generally viewed as healthy and good. However, now a study done in China finds a different result. The 

 A study was just published by researchers at the University of California that reviewed the role of Lactobacillus bacteria in a variety of diseases and conditions. What was surprising was that while we generally think of Lactobacillus bacteria as beneficial, some studies suggest that in certain diseases or conditions they may not be. But it is unknown if in those cases whether they're causing harm or why they are there in increased amounts.
A study was just published by researchers at the University of California that reviewed the role of Lactobacillus bacteria in a variety of diseases and conditions. What was surprising was that while we generally think of Lactobacillus bacteria as beneficial, some studies suggest that in certain diseases or conditions they may not be. But it is unknown if in those cases whether they're causing harm or why they are there in increased amounts.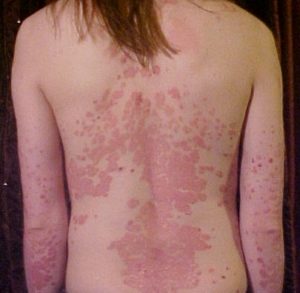
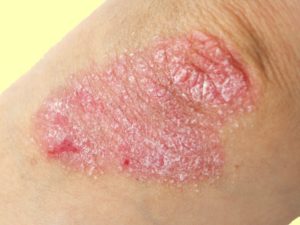 Psoriasis. Credit: Medscape
Psoriasis. Credit: Medscape Did you know that you exchange some skin microbes with the person you live with? A
Did you know that you exchange some skin microbes with the person you live with? A