 In the past year I keep coming across one special gut microbe: Faecalibacterium prausnitzii. This bacteria is considered beneficial and is one of the most prevalent intestinal bacterial species in healthy adults. The reduction of this bacteria in the gut (as measured by analyzing bacteria in fecal samples) is seen in several diseases, including Intestinal Bowel Disease (IBD). This bacteria has also been found to be anti-inflammatory. In other words, you really, really want a healthy population in your gut.
In the past year I keep coming across one special gut microbe: Faecalibacterium prausnitzii. This bacteria is considered beneficial and is one of the most prevalent intestinal bacterial species in healthy adults. The reduction of this bacteria in the gut (as measured by analyzing bacteria in fecal samples) is seen in several diseases, including Intestinal Bowel Disease (IBD). This bacteria has also been found to be anti-inflammatory. In other words, you really, really want a healthy population in your gut.
But now the question is: how does the bacteria get there? And how can you increase it if you have a low population in your gut? It certainly isn't found in any probiotic supplement that I know of. Part of the answer seems to be eating foods with fiber, lots of it, to feed the good microbes. Eat fruits, vegetables, whole grains, seeds, legumes, and nuts.
The following lengthy article discusses the importance of keystone species (F. prausnitzii is one). From Scientific American:
Among Trillions of Microbes in the Gut, a Few Are Special
In the mid-2000s Harry Sokol, a gastroenterologist at Saint Antoine Hospital in Paris, was surprised by what he found when he ran some laboratory tests on tissue samples from his patients with Crohn's disease, a chronic inflammatory disorder of the gut.. But when Sokol did a comparative DNA analysis of diseased sections of intestine surgically removed from the patients, he observed a relative depletion of just one common bacterium, Faecalibacterium prausnitzii. Rather than “bad” microbes prompting disease, he wondered, could a single “good” microbe prevent disease?
Sokol transferred the bacterium to mice and found it protected them against experimentally induced intestinal inflammation. And when he subsequently mixed F. prausnitzii with human immune cells in a test tube, he noted a strong anti-inflammatory response. Sokol seemed to have identified a powerfully anti-inflammatory member of the human microbiota.
Each of us harbors a teeming ecosystem of microbes that outnumbers the total number of cells in the human body by a factor of 10 to one and whose collective genome is at least 150 times larger than our own... The microbiome varies dramatically from one individual to the next and can change quickly over time in a single individual. The great majority of the microbes live in the gut, particularly the large intestine, which serves as an anaerobic digestion chamber.
Independent researchers around the world have identified a select group of microbes that seem important for gut health and a balanced immune system. They belong to several clustered branches of the clostridial group. Dubbed “clostridial clusters,” these microbes are distantly related to Clostridium difficile, a scourge of hospitals and an all too frequent cause of death by diarrhea. But where C. difficile prompts endless inflammation, bleeding and potentially catastrophic loss of fluids, the clostridial clusters do just the opposite—they keep the gut barrier tight and healthy, and they soothe the immune system. Scientists are now exploring whether these microbes can be used to treat a bevy of the autoimmune, allergic and inflammatory disorders that have increased in recent decades, including Crohn's and maybe even obesity.
F. prausnitzii was one of the first clostridial microbes to be identified. In Sokol's patients those with higher counts of F. prausnitzii consistently fared best six months after surgery. After he published his initial findings in 2008, scientists in India and Japan also found F. prausnitzii to be depleted in patients with inflammatory bowel disease... This suggested that whereas different genetic vulnerabilities might underlie the disorder, the path to disease was similar: a loss of anti-inflammatory microbes from the gut. And although Sokol suspects that other good bacteria besides F. prausnitzii exist, this similarity hinted at a potential one-size-fits-all remedy for Crohn's and possibly other inflammatory disorders: restoration of peacekeeping microbes.
One of the questions central to microbiome research is why people in modern society, who are relatively free of infectious diseases, a major cause of inflammation, are so prone to inflammatory, autoimmune and allergic diseases. Many now suspect that society-wide shifts in our microbial communities have contributed to our seemingly hyperreactive immune systems. Drivers of these changes might include antibiotics; sanitary practices that are aimed at limiting infectious disease but that also hinder the transmission of symbiotic microbes; and, of course, our high-sugar, high-fat modern diet. Our microbes eat what we eat, after all. Moreover, our particular surroundings may seed us with unique microbes, “localizing” our microbiota.
A number of studies have found a small but significant correlation between the early-life use of antibiotics and the later development of inflammatory disorders, including asthma, inflammatory bowel disease and, more recently, colorectal cancer and childhood obesity. One explanation for this association might be that sickly people take more antibiotics. Antibiotics are not the cause, in other words, but the result of preexisting ill health. Honda's studies suggest another explanation: antibiotics may deplete the very bacteria that favorably calibrate the immune system, leaving it prone to overreaction.
A number of studies over the years have linked having fewer sanitary amenities in childhood with a lower risk of inflammatory bowel disease in adulthood. And a 2014 study from Aarhus University in Denmark found that among northern Europeans, growing up on a farm with livestock—another microbially enriched environment—halved the risk of being stricken with inflammatory bowel disease in adulthood.
These patterns suggest that perhaps by seeding the gut microbiota early in life or by direct modification of the immune system the environment can affect our risk of inflammatory bowel disease despite the genes we carry. And they raise the question of what proactive steps those of us who do not live on farms can take to increase our chances of harboring a healthy mix of microbes.
One of the more surprising discoveries in recent years is how much the gut microbiota of people living in North America differs from those of people living in rural conditions in Africa and South America. The microbial mix in North America is geared to digesting protein, simple sugars and fats, whereas the mix in rural African and Amazonian environments is far more diverse and geared to fermenting plant fiber. Some think that our hunter-gatherer ancestors harbored even greater microbial diversity in their guts.
What troubles Sonnenburg about this shift is that the bacteria that seem most anti-inflammatory—including the clostridial clusters—often specialize in fermenting soluble fiber...Some hunter-gatherers consumed up to 10 times as much soluble fiber as modern populations, and their bodies likely were flooded with far more fermentation by-products. Our fiber-poor modern diet may have weakened that signal, producing a state of “simmering hyperreactivity,” Sonnenburg says, and predisposing us to the “plagues” of civilization. He calls this problem “starving our microbial self.” We may not be adequately feeding some of the most important members of our microbiota.
Mouse experiments support the idea. Diets high in certain fats and sugars deplete anti-inflammatory bacteria, thin the mucous layer and foster systemic inflammation. ...In rodents, adding fermentable fiber to a diet otherwise high in fat keeps the “good” microbes happy, the mucous layer healthy and the gut barrier intact, and it prevents systemic inflammation. Taken together, these studies suggest that it is not only what is in your food that matters for your health but also what is missing.
The human studies are even more intriguing... Scientists at Catholic University of Louvain in Belgium recently showed that adding inulin, a fermentable fiber, to the diet of obese women increased counts of F. prausnitzii and other clostridial bacteria and reduced that dangerous systemic inflammation...Those without the bacteria did not benefit, which suggests that once species disappear from the “microbial organ,” the associated functions might also vanish. These individuals might not require ecosystem engineering so much as an ecosystem restoration.
 A problem with the Western diet that most of us eat is that it is low in fiber. A diet low in fiber and large amounts of ultraprocessed foods is suspected to play a role in the increasing incidence of intestinal bowel diseases (IBD) in the US and Canada.
A problem with the Western diet that most of us eat is that it is low in fiber. A diet low in fiber and large amounts of ultraprocessed foods is suspected to play a role in the increasing incidence of intestinal bowel diseases (IBD) in the US and Canada.
 The number of people who have Crohn's disease has been rising steadily over the years. Crohn's disease is a debilitating intestinal bowel disease (IBD) which causes severe abdominal pain, diarrhea, weight loss, and fatigue. A number of studies have focused on gut bacteria in those with Crohn's disease and found dysbiosis (microbial community out of whack), but there is disagreement over the cause of Crohn's and the dysbiosis.
The number of people who have Crohn's disease has been rising steadily over the years. Crohn's disease is a debilitating intestinal bowel disease (IBD) which causes severe abdominal pain, diarrhea, weight loss, and fatigue. A number of studies have focused on gut bacteria in those with Crohn's disease and found dysbiosis (microbial community out of whack), but there is disagreement over the cause of Crohn's and the dysbiosis. Two recent studies caught my eye – both reviews of scientific research that looked at the issue of diet and whether it contributes to the development of Intestinal Bowel Disease (IBD), specifically Crohn’s disease and ulcerative colitis. These are chronic inflammatory disorders of the gastrointestinal tract, and which are rapidly increasing in developed countries (over 1 million individuals in the US). The main question is: Does a person’s diet contribute to the development of IBD?
Two recent studies caught my eye – both reviews of scientific research that looked at the issue of diet and whether it contributes to the development of Intestinal Bowel Disease (IBD), specifically Crohn’s disease and ulcerative colitis. These are chronic inflammatory disorders of the gastrointestinal tract, and which are rapidly increasing in developed countries (over 1 million individuals in the US). The main question is: Does a person’s diet contribute to the development of IBD?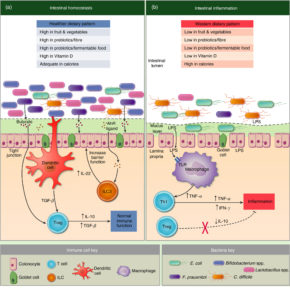
 The use of nanoparticles in foods is increasing every year, but we still know very little about whether they have health risks to humans, especially if one is eating foods with them daily (thus having chronic exposure). The
The use of nanoparticles in foods is increasing every year, but we still know very little about whether they have health risks to humans, especially if one is eating foods with them daily (thus having chronic exposure). The  My last post
My last post  In the past year I keep coming across one special gut microbe: Faecalibacterium prausnitzii. This bacteria is considered beneficial and is one of the most prevalent intestinal bacterial species in healthy adults. The reduction of this bacteria in the gut (as measured by analyzing bacteria in fecal samples) is seen in several diseases, including Intestinal Bowel Disease (IBD). This bacteria has also been found to be anti-inflammatory. In other words, you really, really want a healthy population in your gut.
In the past year I keep coming across one special gut microbe: Faecalibacterium prausnitzii. This bacteria is considered beneficial and is one of the most prevalent intestinal bacterial species in healthy adults. The reduction of this bacteria in the gut (as measured by analyzing bacteria in fecal samples) is seen in several diseases, including Intestinal Bowel Disease (IBD). This bacteria has also been found to be anti-inflammatory. In other words, you really, really want a healthy population in your gut.