 An important study was recently published that documents that when pregnant women are exposed to potentially harmful chemicals in the environment around them (environmental pollutants), many of these chemicals also get transferred to the fetus. Many of the 59 chemicals looked for were detected in the newborn babies' umbilical cords (thus the chemicals had crossed the placenta to the fetus during the pregnancy). Some chemicals were measured in higher levels in the umbilical cord than in the mother (as measured in her blood), while only cadmium appeared in mothers, but not in the umbilical cord (thus there was some protection for the fetus from that particular chemical).
An important study was recently published that documents that when pregnant women are exposed to potentially harmful chemicals in the environment around them (environmental pollutants), many of these chemicals also get transferred to the fetus. Many of the 59 chemicals looked for were detected in the newborn babies' umbilical cords (thus the chemicals had crossed the placenta to the fetus during the pregnancy). Some chemicals were measured in higher levels in the umbilical cord than in the mother (as measured in her blood), while only cadmium appeared in mothers, but not in the umbilical cord (thus there was some protection for the fetus from that particular chemical).
This study did not look at the effects on the fetus and newborn from exposure to all these toxic chemicals - it just found that out of 59 chemicals tested for, the median number was 25 in maternal blood and 17 in umbilical cord blood. Eight of the 59 chemicals analyzed were detected in more than 90 percent of both the maternal and cord blood samples - and these included lead and mercury.
The researchers pointed out that no human studies have examined the developmental and reproductive health effects on the fetus and baby from being exposed to multiple chemicals simultaneously during pregnancy - which of course can have bigger risks and negative effects than being exposed to only one chemical at a time. Other studies have already shown that there are negative health effects (adverse neurodevelopmental effects) from a number of these chemicals, such as PCBs, PBDEs, Pb (lead) and Hg (mercury). Bottom line: No one really knows what these mixtures of chemicals do to the developing baby, but it is known that these chemicals have potential health risks. From Futurity:
Mom’s exposure to toxic chemicals shows up in newborn
Low-income and Latina pregnant women in a recent study had widespread exposure to environmental pollutants. In addition, many of the toxins showed up at even higher levels in their newborns. The study is the first in the United States to measure exposure to 59 toxic chemicals in pregnant women and their newborns.
“Pregnant women in the US are exposed to many harmful industrial chemicals that have been linked to premature birth, low birth weight, and birth defects, but estimates of how efficiently pollutants are transferred from mother to fetus have varied widely,” says Tracey Woodruff, professor of obstetrics, gynecology, and reproductive sciences and the Philip R. Lee Institute for Health Policy Studies at the University of California, San Francisco. “Our findings have found that many chemicals do indeed accumulate in the fetal environment and are absorbed at greater levels by fetuses than by the pregnant women themselves. This may have significant consequences for the growing fetus, since many of these chemicals are known to affect development.”
Researchers measured polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs), polybrominated diphenyl ethers (PBDEs), perfluorinated compounds (PFCs), mercury, and lead, among other chemicals. These industrial pollutants are common in the environment, and in previous studies many have been detected in greater than 99 percent of US pregnant women, according to National Health and Nutrition Examination Survey (NHANES) data.
“Contrary to previous research, we found evidence that several PCBs and OCPs were often higher in umbilical cord samples than in maternal blood samples,” says Rachel Morello-Frosch, professor of environmental science, policy and management at the University of California, Berkeley. The study also found that concentrations of mercury and certain PBDEs were often higher in umbilical cord samples than in maternal samples, and for most PFCs and lead, cord blood concentrations were generally equal to or lower than maternal concentrations, which is consistent with previous research.
Almost 80 percent of the chemicals detected in maternal blood samples were also detected in the umbilical cord blood samples, indicating that they passed through the placenta and entered the fetal environment, where they can pose a health risk to the developing baby. For those chemicals detected in at least 20 paired maternal and umbilical cord samples, 77 percent had significant correlations between maternal and umbilical cord concentrations.
The women in the study were participating in the Chemicals in Our Bodies Study, also referred to as the Maternal and Infant Environmental Exposure Project. Of the women participating in the current study, 95 percent had a combined annual household income of less than $40,000, two-thirds were Latina, and a third were born in Mexico, where they may have had less exposure to environmental toxics like the PBDEs found in flame retardants that have been widely used in the US. This demographic is often not well-represented in larger biomonitoring studies, such as NHANES, that form the basis of most of what is known about pregnant women’s exposure to environmental toxics nationally.
The study also provides a first indication of how several different classes of environmental chemicals found in a pregnant woman’s blood are also present in the newborn....From 2010 to 2011, researchers collected maternal blood samples from 77 pregnant women at Zuckerberg San Francisco General. Once they delivered their babies, researchers collected umbilical cord blood samples from 65 of these women. Of those samples tested for all 59 chemicals, the median number was 25 in maternal blood and 17 in umbilical cord blood. Eight of the 59 chemicals analyzed were detected in more than 90 percent of both the maternal and cord blood samples.
 Interesting, yet disturbing, research found microplastics in human placentas and also that the microplastic contamination of placentas has been rising steadily since 2006. During this time global plastic production has also increased.
Interesting, yet disturbing, research found microplastics in human placentas and also that the microplastic contamination of placentas has been rising steadily since 2006. During this time global plastic production has also increased.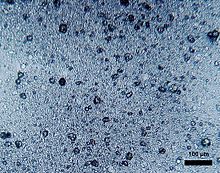

 Most people know that heavy drinking of alcohol, smoking marijuana, cigarette smoking, and using methamphetamine during pregnancy should be avoided because they can have negative effects on the developing fetus. However,
Most people know that heavy drinking of alcohol, smoking marijuana, cigarette smoking, and using methamphetamine during pregnancy should be avoided because they can have negative effects on the developing fetus. However,