 Another article commenting that increasing the amount of dietary fiber eaten by people eating a typical Western diet (which is low in fiber) will improve their gut microbiome (community of microbes). Research is finding that the added dietary fiber is food (nutrition) for microbes in the gut, and eating additional fiber daily will help restore or increase bacterial diversity, which then should lead to health benefits. Note: Easy ways to increase dietary fiber are increasing intake of whole grains, legumes, nuts, seeds, fruits, and vegetables. Think of food writer Michael Pollan's advice: "Eat food. Not too much. Mostly plants."
Another article commenting that increasing the amount of dietary fiber eaten by people eating a typical Western diet (which is low in fiber) will improve their gut microbiome (community of microbes). Research is finding that the added dietary fiber is food (nutrition) for microbes in the gut, and eating additional fiber daily will help restore or increase bacterial diversity, which then should lead to health benefits. Note: Easy ways to increase dietary fiber are increasing intake of whole grains, legumes, nuts, seeds, fruits, and vegetables. Think of food writer Michael Pollan's advice: "Eat food. Not too much. Mostly plants."
Researchers feel that fiber intake needs to be increased to more than current dietary guidelines, and that beneficial effects to the microbiome starts to occur rapidly (within 2 weeks) of changing to a higher fiber diet. This post from January 21, 2016 discussed the Sonnenburg research on gut microbe depletion (from a low fiber diet), and this April 28, 2015 post discussed the O'Keefe research (changing the diet has big effect on colon cancer risk) - both studies are mentioned below. See the page Feeding Your Gut Microbes for more information. From Science Daily:
Can more fiber restore microbiome diversity?
Scientists are pushing to restore human health in Western countries by changing our diet to restore the microbial species lost over the evolution of Western diet. Researchers advocate for strategically increasing dietary fiber intake as one path forward in regaining microbial biodiversity.
Insufficient nutrients for our gut microbes have been linked to a loss of certain beneficial bacterial species in industrialized societies and are likely impacting our immunological and metabolic health, although more data is needed. For example, most Westerners consume half of the amount of dietary fiber recommended by dietary guidelines, which nutritionists refer to as the "fiber gap," which is a problem because dietary fiber is the primary source of nutrition (e.g., carbohydrates) accessible to gut bacteria in humans.
"The idea to boost fiber levels is not new," says Jens Walter of the University of Alberta, Canada. "However, depletion of the microbiome adds a new perspective to this low-fiber Western diet that we are currently eating." Earlier this year, Stanford University's Justin Sonnenburg found that mice fed a typical Western diet (high in fat and carbohydrates and low in fiber) transferred a lower diversity of beneficial microbial species to future generations. The re-introduction of the microbes' preferred fiber at that stage did not result in a return of some (good) species, indicating that extinctions had occurred in only a few generations.
Walter and co-author Edward Deehan, his PhD student, are concerned that a dramatic shift away from a diet similar to the one under which the human-microbiome symbiosis evolved is a key factor in the rise of non-communicable disorders like obesity. "There is a lot of epidemiological evidence that fiber is beneficial, and food products containing dietary fiber have FDA-approved health claims for both colon cancer and coronary heart disease. There is also quite a bit of clinical evidence (although it is less consistent)," Walter says. "The most pressing issue at the moment that neither consumption of fiber in society nor the doses used in clinical research are high enough."
People living in non-industrialized societies have an average intake of fiber that is much higher than the low norms of Western societies. The authors note the recent work from the Stephen J.D. O'Keefe lab in Nature Communications in which modern African-Americans were given a traditional South-African diet that contained 55 grams of daily dietary fiber and had improved markers for colon cancer within two weeks.
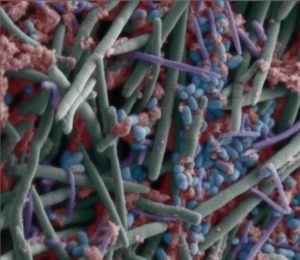


 And what is a health-promoting diet?
And what is a health-promoting diet?
 Recent research found that flare-ups of the disease lupus involves microbial changes in the gut microbiome. The gut microbiome is the community of microbes (fungi, bacteria, viruses) living in the small intestine.
Recent research found that flare-ups of the disease lupus involves microbial changes in the gut microbiome. The gut microbiome is the community of microbes (fungi, bacteria, viruses) living in the small intestine. It has been known for a while that eating fermented foods and high fiber foods is healthy for us. A recent
It has been known for a while that eating fermented foods and high fiber foods is healthy for us. A recent  Uh oh... Glyphosate is the most widely used herbicide (plant-killer) in the world, and its pervasive use may be harming our gut microbiomes. Glyphosate (which is in Roundup) is used not only as a weed-killer, but also applied to glyphosate resistant genetically engineered (GE) crops such as soy, canola, corn, and also right before harvest (
Uh oh... Glyphosate is the most widely used herbicide (plant-killer) in the world, and its pervasive use may be harming our gut microbiomes. Glyphosate (which is in Roundup) is used not only as a weed-killer, but also applied to glyphosate resistant genetically engineered (GE) crops such as soy, canola, corn, and also right before harvest ( Researchers are starting to raise concerns about routine daily intake of probiotics for "gut health". Much is still unknown, but problems are starting to appear. A healthy gut contains hundreds of species (bacteria, fungi, viruses), and taking megadoses of a few species (a probiotic supplement) can overwhelm the normal gut microbial community. A healthy gut is one with a greater diversity of species, not just some species.
Researchers are starting to raise concerns about routine daily intake of probiotics for "gut health". Much is still unknown, but problems are starting to appear. A healthy gut contains hundreds of species (bacteria, fungi, viruses), and taking megadoses of a few species (a probiotic supplement) can overwhelm the normal gut microbial community. A healthy gut is one with a greater diversity of species, not just some species.