 Many probiotic manufacturers say that their product has all sorts of wonderful health benefits in people eating that particular probiotic, but is the evidence there? Finally, now there is a review of the best existing studies looking at whether probiotics have any effect on the gut bacteria of healthy, normal individuals. In other words, are the probiotics even staying there (to have some beneficial effect) or do they just "pass through" without leaving anything behind?
Many probiotic manufacturers say that their product has all sorts of wonderful health benefits in people eating that particular probiotic, but is the evidence there? Finally, now there is a review of the best existing studies looking at whether probiotics have any effect on the gut bacteria of healthy, normal individuals. In other words, are the probiotics even staying there (to have some beneficial effect) or do they just "pass through" without leaving anything behind?
The main finding: only one study out of 7 found any lasting effect on gut microbes (in healthy individuals) from the probiotics which had been ingested daily over varying times, but typically for one month. Note: RCTs are randomly controlled trials, which are the best way to test whether something has an effect - because people are randomly assigned to a group. In these studies no one knew who was getting a placebo (e.g., received capsule without the probiotic) or the probiotic (e.g., in the capsule) - this eliminates self-selection and bias. But perhaps the bacteria strains tested were the wrong ones? Or the time period wasn't long enough or the bacteria weren't given in sufficient amounts? Also, studies didn't test multi-strain probiotics (which people commonly take), but only 1 or 2 species of bacteria.
However, other research has shown benefits from probiotics in individuals with "dysbiosis" (microbial communities out of whack) or certain illnesses. It'll be interesting to see what further research finds. These are still early days in this research. From MedicalXpress:
Do probiotics have an effect on healthy adults? It's too early to tell
There is little evidence to support any consistent effect of probiotics on the gut microbiota of healthy individuals, according to a systematic review published in the open access journal Genome Medicine. The World Health Organization defines probiotics as live microorganisms which confer a health benefit to the host if administered in adequate amounts and probiotics products are often marketed toward the general population. However, evidence for their effects on bacteria living in the guts of healthy adults remains elusive.
The study by researchers at the Novo Nordisk Foundation Center for Basic Metabolic Research at the University of Copenhagen is a systematic review of seven randomized controlled trials (RCTs) investigating the effect of probiotic products on the fecal microbiota of healthy adults.
Nadja Buus Kristensen, PhD student and junior author, said: "According to our systematic review, no convincing evidence exists for consistent effects of examined probiotics on fecal microbiota composition in healthy adults, despite probiotic products being consumed to a large extent by the general population."...The authors found that of the seven original RCTs included in the study, only one observed significantly greater changes in the bacterial species composition of the fecal microbiota in individuals who consumed probiotics compared to those who did not.
Also, an international consensus on what defines a normal or healthy fecal microbial community is lacking....Study participants across the seven original RCTs included in this review were healthy adults between 19 and 88 years of age. Numbers of individuals ranged from 21 to 81 and the proportion of women was between 50 and 100%. Probiotic products were administered as biscuits, milk-based drinks, sachets, or capsules for periods of 21 to 42 days.
Oluf Pedersen, professor at the University of Copenhagen and senior author of the paper said: "While there is some evidence from previous reviews that probiotic interventions may benefit those with disease-associated imbalances of the gut microbiota, there is little evidence of an effect in healthy individuals.

 E. coli bacteria in urine sample
E. coli bacteria in urine sample breast cancer cells
breast cancer cells prostate cancer cell
prostate cancer cell large intestine
large intestine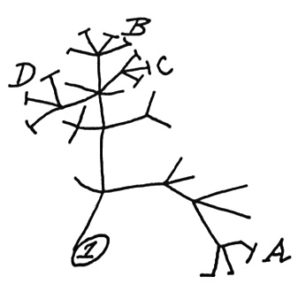 In 1837, Charles Darwin sketched a simple tree of life (shown left) to illustrate the idea that all living things share a common ancestor. Ever since then, scientists have been adding names to the
In 1837, Charles Darwin sketched a simple tree of life (shown left) to illustrate the idea that all living things share a common ancestor. Ever since then, scientists have been adding names to the 
 Another article commenting that
Another article commenting that  Toxoplasma gondii tissue cyst in a mouse brain
Toxoplasma gondii tissue cyst in a mouse brain  The eye has a normal community of microbes or eye microbiome, just like other body sites (i.e., the gut, the sinuses, the mouth). This community of bacteria is thought to offer resistance from invaders (such as pathogenic bacteria).
The eye has a normal community of microbes or eye microbiome, just like other body sites (i.e., the gut, the sinuses, the mouth). This community of bacteria is thought to offer resistance from invaders (such as pathogenic bacteria). The possibility of giving microbes in the future (whether bacteria, viruses, or fungi) to treat cancer is amazing. Of course big pharma is pursuing this line of research, which is called immunotherapy (stimulating the body's ability to fight tumors). The Bloomberg Business article discusses a number of big pharma companies entering the field and their main focus. The study in the journal Science finding that giving common beneficial bacteria (Bifidobacterium breve and Bifidobacterium longum) to mice to slow down melanoma tumor growth is a first step. The researchers themselves said that the 2 common beneficial bacteria species exhibited anti-tumor activity in the mice and was as effective as an immunotherapy in controlling the growth of skin cancer. But note that the bacteria needed to be live. Stay tuned....
The possibility of giving microbes in the future (whether bacteria, viruses, or fungi) to treat cancer is amazing. Of course big pharma is pursuing this line of research, which is called immunotherapy (stimulating the body's ability to fight tumors). The Bloomberg Business article discusses a number of big pharma companies entering the field and their main focus. The study in the journal Science finding that giving common beneficial bacteria (Bifidobacterium breve and Bifidobacterium longum) to mice to slow down melanoma tumor growth is a first step. The researchers themselves said that the 2 common beneficial bacteria species exhibited anti-tumor activity in the mice and was as effective as an immunotherapy in controlling the growth of skin cancer. But note that the bacteria needed to be live. Stay tuned.... A thought-provoking
A thought-provoking 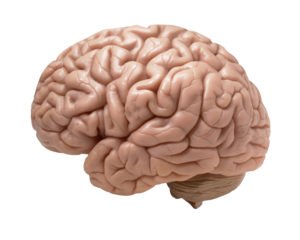 Two articles about the link between Alzheimer's disease (AD) and microbes this past week: a study linking periodontal disease and Alzheimer's, and the other a journal editorial (written by an international team of 31 researchers) suggesting that we need to more closely look at the role of microbes in Alzheimer's disease, especially herpes virus, chlamydia and spirochaete bacteria.
Two articles about the link between Alzheimer's disease (AD) and microbes this past week: a study linking periodontal disease and Alzheimer's, and the other a journal editorial (written by an international team of 31 researchers) suggesting that we need to more closely look at the role of microbes in Alzheimer's disease, especially herpes virus, chlamydia and spirochaete bacteria._lores.jpg) Borrelia burgdorferi Credit: CDC
Borrelia burgdorferi Credit: CDC