 A recent study tested a variety of probiotic (beneficial) Lactobacillus and Bifidobacteria species of bacteria as a treatment for chronic sinusitis. Unfortunately, it found that the microbes tested had NO effect on chronic sinusitis symptoms. It was a nice study conducted in Sweden, with 21 people with chronic sinusitis (but without nasal polyps) randomly assigned to receive a nasal spray (that they used 2 x daily for 14 days) containing either a mixture of 13 bacteria or a "sham" nasal spray. No one knew who received what, and then after a few weeks they did a crossover - meaning who got what was switched for another 2 weeks.
A recent study tested a variety of probiotic (beneficial) Lactobacillus and Bifidobacteria species of bacteria as a treatment for chronic sinusitis. Unfortunately, it found that the microbes tested had NO effect on chronic sinusitis symptoms. It was a nice study conducted in Sweden, with 21 people with chronic sinusitis (but without nasal polyps) randomly assigned to receive a nasal spray (that they used 2 x daily for 14 days) containing either a mixture of 13 bacteria or a "sham" nasal spray. No one knew who received what, and then after a few weeks they did a crossover - meaning who got what was switched for another 2 weeks.
But...the main finding is that after 14 days of using the nasal sprays, there was no improvement in either group, no improvement in symptoms, no effect on the sinus "microbial flora", and no effect on inflammation. In fact, 2 individuals wound up taking antibiotics while testing the bacteria nasal spray. In other words, a big fat zero.
The bacteria tested were what the researchers called a honeybee lactic acid (LAB) microbiome, with both Lactobacillus and Bifidobacteria species: Lactobacillus apinorum, L. mellifer, L. mellis, L. kimbladii, L. melliventris, L. helsingborgensis, L. kullabergensis, L. kunkeei, L. apis, Bifidobacterium asteroides, B. coryneforme, Bifidobacterium Bin7N, and Bifidobacterium Hma3N. These species are not typically found in probiotic supplements.
Why did they choose those strains of bacteria? Because "in vitro" testing (meaning in a test tube or culture dish) suggested that they would be effective against the pathogenic bacteria frequently found in chronic sinusitis (that they were antimicrobial). But real world testing in actual humans in this study showed that those specific Lactobacillus and Bifidobacteria microbes had no effect on sinusitis symptoms. Their premise was good - that the sinus microbiome was "disturbed" or out of whack (dysbiosis) in chronic sinusitis, but unfortunately they chose the wrong bacteria to test as a treatment.
The SNOT-22 questionnaire that asked questions of sinusitis sufferers at several points in the study to see if there was improvement in sinusitis symptoms, is one typically given to those with chronic sinusitis. [By the way, when reviewing the questionnaire, I realized it left out some major sinusitis symptoms such as "gagging on phlegm", "waking up with sore throat", "teeth hurt", "headache" - all of which are frequently mentioned by many contacting me, and which I remember well from pre-L. sakei days. In other words - it is incomplete, yet it is the questionnaire typically used to assess quality of life and symptoms for those with chronic sinusitis.]
The researchers end the journal article by stating "Further studies are warranted to explore whether other tentative probiotic assemblages [other bacterial species] can confer positive health effects to patients suffering from inflammatory conditions of the upper airways." Huh... If only they had asked... I've been writing about Lactobacillus sakei as an excellent treatment for chronic sinusitis since 2013 (based on results of Abreu et al study), and I've been getting positive feedback from others about L. sakei since early 2014. For those who find that L. sakei works as a sinusitis treatment, the results seem miraculous - typically with major improvement within a few days. (Please note: Perhaps other microbes may also work as a sinusitis treatment.) Excerpts from Laryngoscope Investigative Otolaryngology:
A locally disturbed commensal microbiome might be an etiological factor in chronic rhinosinusitis (CRS) in general and in CRS without nasal polyps (CRSsNP) in particular. Lactic acid bacteria (LAB) have been suggested to restore commensal microbiomes. A honeybee LAB microbiome consisting of various lactobacilli and bifidobacteria have been found potent against CRS pathogens in vitro. Recently, we examined effects of single nasal administrations of this microbiome in healthy subjects and found it inert. In this study, we examined effects of repeated such administrations in patients with CRSsNP.
The study was of a randomized, double‐blinded, crossover, and sham‐controlled design. Twenty patients received 2 weeks' treatment administered using a nasal spray‐device. The subjects were monitored with regard to symptoms (SNOT‐22 questionnaire, i.e., the primary efficacy variable), changes to their microbiome, and inflammatory products (IL‐6, IL‐8, TNF‐, IL‐8,a, and MPO) in nasal lavage fluids.
Results: Neither symptom scores, microbiological explorations, nor levels of inflammatory products in nasal lavage fluids were affected by LAB (c.f. sham). Conclusion: Two weeks' nasal administration of a honeybee LAB microbiome to patients with CRSsNP is well tolerated but affects neither symptom severity nor the microbiological flora/local inflammatory activity.
In this study, involving patients with well‐defined CRSsNP, we demonstrate that repeated nasal administration of a LAB microbiota composed of several species of lactobacilli and bifidobacteria over 2 weeks neither affects symptoms as assessed by SNOT‐22 questionnaire nor the bacterial composition or the inflammatory activity in the nasal cavity. The observations are of relevance to the evaluation of topical LAB treatment in the management of upper respiratory tract conditions such as CRS.

 A study was just published by researchers at the University of California that reviewed the role of Lactobacillus bacteria in a variety of diseases and conditions. What was surprising was that while we generally think of Lactobacillus bacteria as beneficial, some studies suggest that in certain diseases or conditions they may not be. But it is unknown if in those cases whether they're causing harm or why they are there in increased amounts.
A study was just published by researchers at the University of California that reviewed the role of Lactobacillus bacteria in a variety of diseases and conditions. What was surprising was that while we generally think of Lactobacillus bacteria as beneficial, some studies suggest that in certain diseases or conditions they may not be. But it is unknown if in those cases whether they're causing harm or why they are there in increased amounts.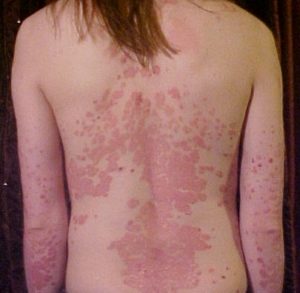
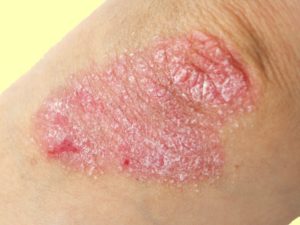 Psoriasis. Credit: Medscape
Psoriasis. Credit: Medscape Could something as simple as giving a probiotic and a sugar for 7 days prevent sepsis in babies? Sepsis is a life-threatening infection that is a HUGE problem in developing countries such as India. It is a major cause of death in babies throughout the world, even with antibiotic treatment. So this new research (done in India) finding that giving newborn babies a probiotic plus the sugar fructooligosaccharide (FOS) for only one week had the result of lowering the incidence of sepsis and death by 40%, and also infections is huge news. A game changer.
Could something as simple as giving a probiotic and a sugar for 7 days prevent sepsis in babies? Sepsis is a life-threatening infection that is a HUGE problem in developing countries such as India. It is a major cause of death in babies throughout the world, even with antibiotic treatment. So this new research (done in India) finding that giving newborn babies a probiotic plus the sugar fructooligosaccharide (FOS) for only one week had the result of lowering the incidence of sepsis and death by 40%, and also infections is huge news. A game changer. Amazing if this holds up in larger studies - a treatment for peanut allergy! As the
Amazing if this holds up in larger studies - a treatment for peanut allergy! As the  An amazing new study is about to start in Sweden - this study will see if "snot transplants" work for the treatment for chronic sinusitis! Will this turn out to be a permanent treatment? While
An amazing new study is about to start in Sweden - this study will see if "snot transplants" work for the treatment for chronic sinusitis! Will this turn out to be a permanent treatment? While  Last week a person told an
Last week a person told an  Could this be true? Probiotics for seasonal allergies? A
Could this be true? Probiotics for seasonal allergies? A