
Soon there may be an oral vaccine for recurrent urinary tract infections (UTIs). That is wonderful news for millions of women suffering from frequent urinary tract infections, who wind up taking repeated courses of antibiotics. Yet they keep getting UTIs.
In a small study, 54% of the persons who took the vaccine remained UTI free for the entire 9 year follow-up period, with the average UTI free period in the group (72 women and 17 men) of 4 1/2 years. The researchers said the results were a "game-changer" for the study participants.
In this study, the vaccine was given "off label" in the UK. 40% of the group had repeated doses after 1 or 2 years. Please view the study as having preliminary results, especially because the study did not have a control group that didn't receive the vaccine. There were no notable adverse effects from the vaccine .
The vaccine, called Uromune (MV140), was developed by Immunotek in Spain. The vaccine is composed of inactivated whole bacteria commonly associated with UTIs: E.coli, K. pneumoniae, P. vulgaris, and E. faecalis. It is taken every day - 2 sprays of a pineapple flavored liquid under the tongue for three months.
The vaccine is not approved by the FDA in the US at this time. It is currently pending approval in Canada and available off-license in 26 countries, including Mexico. Clinical trials are now going on. It has been in use for several years (more than 40,000 patients).
 What to do now? While you're waiting for US FDA vaccine approval, why not try D-mannose for UTIs? It's non-prescription, safe, available as capsule or powder, works well for most species implicated in UTIs (such as E. coli), and readily available. Studies support its use as either a preventive or a treatment for UTIs, and as a replacement for antibiotics.
What to do now? While you're waiting for US FDA vaccine approval, why not try D-mannose for UTIs? It's non-prescription, safe, available as capsule or powder, works well for most species implicated in UTIs (such as E. coli), and readily available. Studies support its use as either a preventive or a treatment for UTIs, and as a replacement for antibiotics.
The vaccine results were recently presented at a European medical conference. From Medical Xpress: Oral vaccine for UTI is potential alternative to antibiotics, finds 9-year study
Recurrent Urinary Tract Infections (UTIs) can be prevented for up to nine years in more than half of people given an oral spray-based vaccine and is a potential alternative to antibiotic treatments, finds research. ...continue reading "Oral Vaccine for Recurrent Urinary Tract Infections"

 It has been known for a while that getting a good night's sleep after getting vaccinated results in a stronger immune response. Twice as strong response! A recent
It has been known for a while that getting a good night's sleep after getting vaccinated results in a stronger immune response. Twice as strong response! A recent 
 The possibility of a vaccine for helping the body fight cancer just got one step closer. A vaccine that targets a specific type of usually incurable brain cancer called "diffuse glioma" has had very good results in
The possibility of a vaccine for helping the body fight cancer just got one step closer. A vaccine that targets a specific type of usually incurable brain cancer called "diffuse glioma" has had very good results in 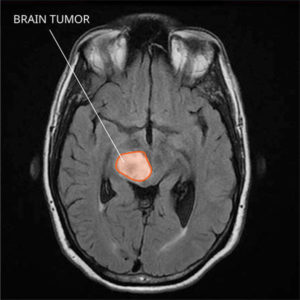
 There is much debate over whether pregnant women should get a COVID-19 vaccine. This is because studies of pregnant women receiving vaccinations have not been done, and so risks and possible harms (if any) are unknown. But what is known is that pregnant women are at higher risk for pregnancy complications if they get COVID-19 (e.g. increased risk of preterm labor and stillbirth).
There is much debate over whether pregnant women should get a COVID-19 vaccine. This is because studies of pregnant women receiving vaccinations have not been done, and so risks and possible harms (if any) are unknown. But what is known is that pregnant women are at higher risk for pregnancy complications if they get COVID-19 (e.g. increased risk of preterm labor and stillbirth).