If taking Clostridia as a probiotic for food allergies works, it would be amazing for food allergy sufferers. Very exciting research. From Time:
The Bacteria That May One Day Cure Food Allergies
Every round of antibiotics a person takes will wipe out strains of bacteria inside the body, some of which are eliminated forever. Considering how early and how often antibiotics are administered to kids—coupled with our increasingly antimicrobial lifestyles—we’ve become more prone to allergies and other ailments, the hygiene hypothesis goes. There’s no cure for food allergies, just lifestyle adjustments and abstention. But Nagler and her team may have the germ of an idea for treatment using gut bacteria, according to a new mice study published in the Proceedings of the National Academy of Sciences.
The team dosed two groups of mice with peanut allergens. One group of mice had been bred to be entirely without gut germs; the other group had sparsely populated gut bacteria due to treatment with antibiotics. Both groups of mice had higher levels of the allergen in their bloodstream compared to mice with healthy gut-bacteria populations.
After giving those same mice a mix that contained the bacteria strain Clostridia, their allergen levels plummeted. Infusing the mice with another group of intestinal bacteria, Bacteroides, didn’t help—so the researchers think the effect is special to Clostridia. “These bacteria are very abundant and they reside very close to the epithelial lining, so they’re in intimate contact with the immune system,” Nagler says.
Next, they’ll transfer gut bacteria from food-allergic infants and healthy infants into germ-free mice, Nagler says. “If we give back Clostridia to a mouse that has the bacteria of an allergic child, can we now reverse susceptibility in that mouse?”
This is a more in-depth article of the research. From Science Daily:
Gut bacteria that protect against food allergies identified
The presence of Clostridia, a common class of gut bacteria, protects against food allergies, a new study in mice finds. The discovery points toward probiotic therapies for this so-far untreatable condition. Food allergies affect 15 million Americans, including one in 13 children, who live with this potentially life-threatening disease that currently has no cure, researchers note.
Although the causes of food allergy -- a sometimes deadly immune response to certain foods -- are unknown, studies have hinted that modern hygienic or dietary practices may play a role by disturbing the body's natural bacterial composition. In recent years, food allergy rates among children have risen sharply -- increasing approximately 50 percent between 1997 and 2011 -- and studies have shown a correlation to antibiotic and antimicrobial use.
"Environmental stimuli such as antibiotic overuse, high fat diets, caesarean birth, removal of common pathogens and even formula feeding have affected the microbiota with which we've co-evolved," said study senior author Cathryn Nagler, PhD, Bunning Food Allergy Professor at the University of Chicago. "Our results suggest this could contribute to the increasing susceptibility to food allergies."
To test how gut bacteria affect food allergies, Nagler and her team investigated the response to food allergens in mice. ...This sensitization to food allergens could be reversed, however, by reintroducing a mix of Clostridia bacteria back into the mice. Reintroduction of another major group of intestinal bacteria, Bacteroides, failed to alleviate sensitization, indicating that Clostridia have a unique, protective role against food allergens.
To identify this protective mechanism, Nagler and her team studied cellular and molecular immune responses to bacteria in the gut. Genetic analysis revealed that Clostridia caused innate immune cells to produce high levels of interleukin-22 (IL-22), a signaling molecule known to decrease the permeability of the intestinal lining.
While complex and largely undetermined factors such as genetics greatly affect whether individuals develop food allergies and how they manifest, the identification of a bacteria-induced barrier-protective response represents a new paradigm for preventing sensitization to food. Clostridia bacteria are common in humans and represent a clear target for potential therapeutics that prevent or treat food allergies.

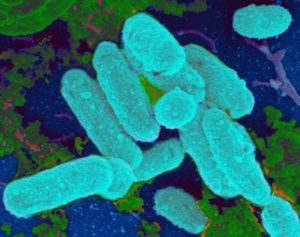 Even though this study was done in a laboratory, it gives further support for the treatment of sinusitis with bacteria and other microbes. And it could help explain why repeated courses of antibiotics don't "cure" many chronic infections - because biofilms filled with pathogenic bacteria are signs of microbial communities out-of-whack. Which is why my family's successful chronic sinusitis treatment with kimchi (juice) containing Lactobacillus sakei is all the more impressive. From Science Daily:
Even though this study was done in a laboratory, it gives further support for the treatment of sinusitis with bacteria and other microbes. And it could help explain why repeated courses of antibiotics don't "cure" many chronic infections - because biofilms filled with pathogenic bacteria are signs of microbial communities out-of-whack. Which is why my family's successful chronic sinusitis treatment with kimchi (juice) containing Lactobacillus sakei is all the more impressive. From Science Daily: I spent time this past week searching the medical literature (US National Library of Medicine - Medline/PubMed) for the latest in sinusitis research. I wish I could tell you that amazing research has been happening recently, especially with the sinus microbiome (which could mean treating sinusitis with microbes), but I was disappointed. Really disappointed.
I spent time this past week searching the medical literature (US National Library of Medicine - Medline/PubMed) for the latest in sinusitis research. I wish I could tell you that amazing research has been happening recently, especially with the sinus microbiome (which could mean treating sinusitis with microbes), but I was disappointed. Really disappointed. Excerpts from a very interesting NPR interview with Dr. Martin Blaser and his views on the human microbiome. The big take-away: our modern life-style is not good for the gut microbiome. His recently published book is Missing Microbes: How the Overuse of Antibiotics is Fueling Our Modern Plagues.
Excerpts from a very interesting NPR interview with Dr. Martin Blaser and his views on the human microbiome. The big take-away: our modern life-style is not good for the gut microbiome. His recently published book is Missing Microbes: How the Overuse of Antibiotics is Fueling Our Modern Plagues.